[More enlargement]
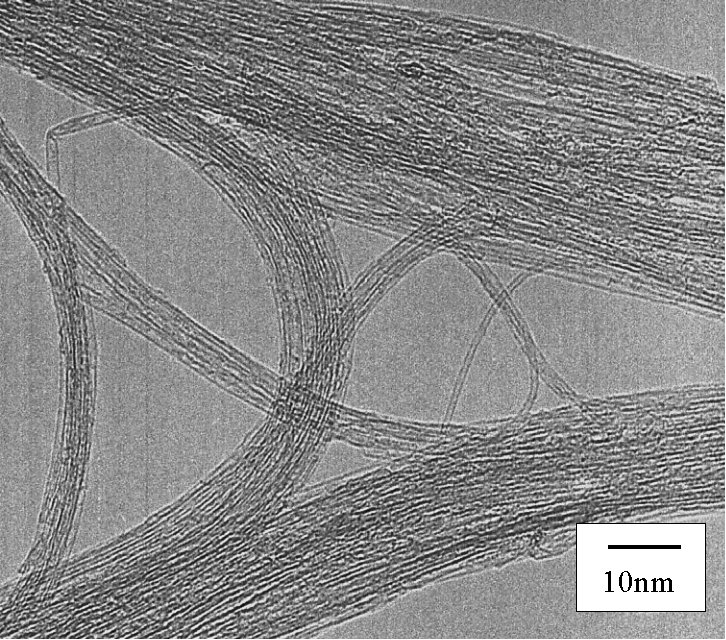
No metal, no amorphous, no MWNTs
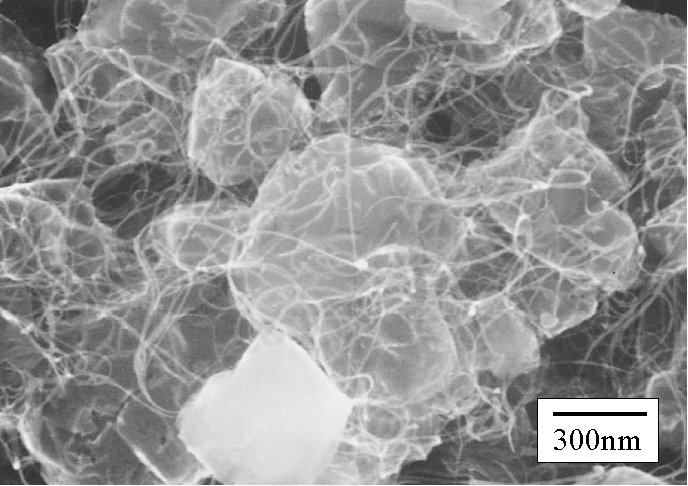
Bundles of SWNTs are observed l
ike web between zeolite particles
Updated: '02/10/24
TEM & SEM observation of AS-GROWN sample
 |
 |
 |
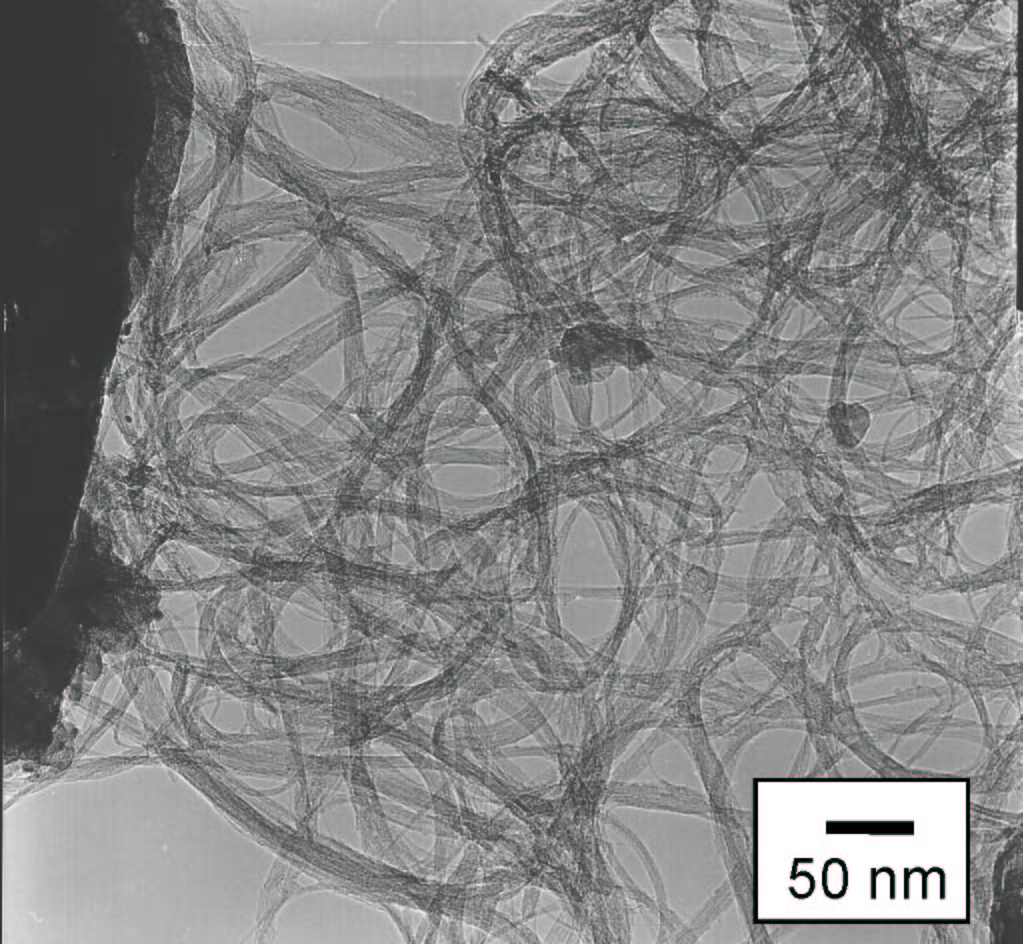
| High-Resolution TEM [Click for enlargement] [More enlargement] |
TEM [Click for enlargement] No metal, no amorphous, no MWNTs |
SEM [Click for enlargement] Bundles of SWNTs are observed l ike web between zeolite particles |
TEM and SEM Pictures of As Grown Sample!
SWNTs generated from alcohol CCVD method
(Ethanol, 800 degC).
For details read the following paper.
S. Maruyama, R. Kojima, Y. Miyauchi, S. Chiashi
and M. Kohno, Chem. Phys. Lett., (2002),
vol. 360, no. 3-4, pp. 229-234.
Resonant Raman Scattering of AS-GROWN sample
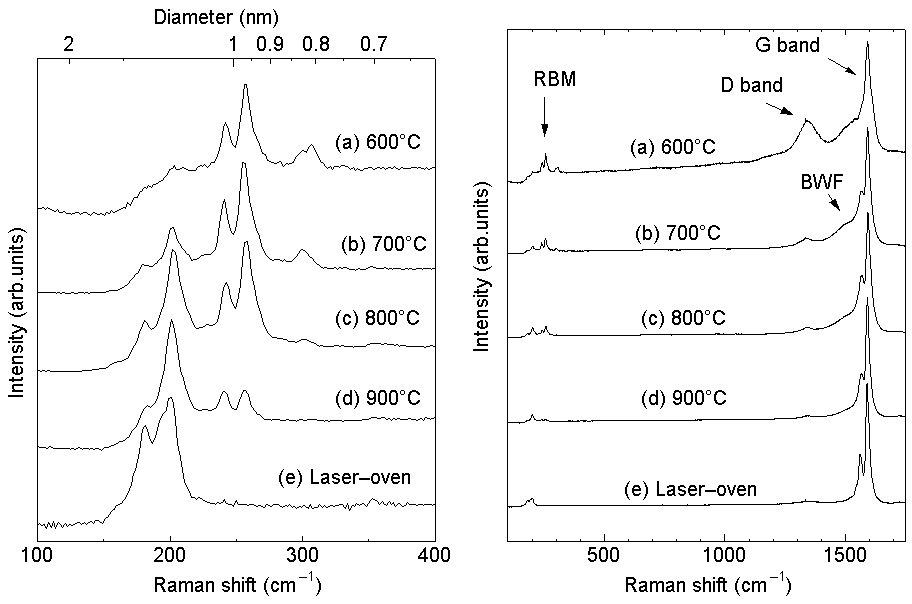
Raman Scattering of As-grown samples with
different reaction temperature.
Excitation 488 nm.
The Resonant features of SWNTs are explained
in the following page.
http://www.photon.t.u-tokyo.ac.jp/~maruyama/kataura/kataura.html.
Experimental Apparatus & Procedure
While we are trying the nanotube generation
from C60 as carbon source, we accidentally
found that very pure SWNTs were made from
ethanol used for the solvent of catalyst
metals. Here, the Fe/Co metal catalysts were
supported with Y-type zeolite according to
the technique developed by Professor Shinohara
at Nagoya University. Our technique is just
very simple as follows.
(1) Catalyst on a quartz boat is set in the
quartz tube in the electric oven.
(2) While heating up the oven, Ar flow is
kept.
(3) At desired temperature, once evacuate
the quartz tube with the mechanical vacuum
pump.
(4) Introduce alcohol vapor flow from the
room temperature reservoir.

[Click for enlargement]
Alcohol catalytic CVD apparatus
Generation Mechanism
This simulation is the image of CCVD growth
of SWNTs from hydrocarbons.
In the case of alcohol, OH radical is left
after the dissociation.
This OH reacts with a carbon with a dangling
bond (Orange Molecules).
Hence, the soruce of formation of amorphous
carbon or MWNTs is prohibited at
the early stage.
Since the selection of SWNTs is through this
chemical reaction, the low-temperature generation
is possible.
Molecular dynamics simulation by Y. Shibuta and S. Maruyama
Ni particle (108 atoms) of about 1.2 nm diameter.
Potentials:
Covalent bond between C-C:Brenner potential
Van der Waals Force: Lennard-Jones potential
C-Ni, Ni-Ni: Original Potential from
Y. Yamaguchi and S. Maruyama, Euro. Phys. J. D, (1999), vol. 9, pp. 385-388.
Carbon atoms (Hydrocarbon molecules) can make covalent bonding only when both atoms were attaching to the surface of catalytic metals.