ICHMT Symposium Molecular & Microscale Heat Transfer,
Yokohama, Dec. 1-5, 1996.
ABSTRACT. The formation mechanism
of fullerene, a new type of carbon molecule with hollow caged
structure, was studied using the molecular dynamics method with
the simplified classical potential function. The clustering process
starting from isolated carbon atoms was simulated under controlled
temperature condition. Here, translational, rotational and vibrational
temperatures of each cluster were controlled to be equilibrium.
The structure of clusters obtained after enough calculation depended
on the controlled temperature Tc, yielding to graphitic
sheet for Tc < 2600 K, fullerene-like caged structure
for 2600 K < Tc < 3500 K, and chaotic 3-dimensional
structure for Tc > 3500 K. Through the detailed
trace of precursors, it was revealed that the key feature of the
formation of the caged structure was the chaotic 3-dimensional
cluster of 40 to 60 atoms which had large vibrational energy.
On the other hand, when precursors were kept under lower vibrational
energy, the successive growth of 2-dimensional graphitic structure
was observed. Since the time scale of the simulation was compressed,
the annealing process of each cluster was virtually omitted. In
order to examine this effect, an imperfect C60 obtained
from the similar simulation was annealed at 2500 K for 50 ns without
collisions. The perfect Buckminsterfullerene C60 was
finally obtained after successive Stone-Wales transformations.
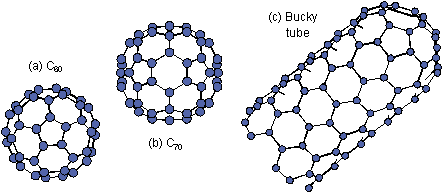
Existence of soccer-ball structured
C60 shown in Fig. 1.1 (a) was demonstrated by Kroto
et al. (1985) through their time-of-flight mass spectra
of carbon clusters generated by the laser-vaporization supersonic-nozzle
technique. They observed a prominent C60+
signal and the complete lack of odd-numbered clusters. They named
the cluster C60 of truncated icosahedron Buckminsterfullerene
since the beautiful network structure resembled the famous geodesic
dome designed by Buckminster Fuller. Since mass spectra of positive
carbon clusters exhibited only even numbered clusters in the range
of C30 to at least C600 [Rohlfing et
al. (1984) and Maruyama et al. (1991)], we could speculate
that all of those clusters had polyhedral structure. Carbon clusters
with such hollow close-caged structure are called fullerene [Fig.
1.1]. In 1990, discoveries of simple techniques for macroscopic
generation and isolation of fullerene by Krtschmer et al.
(1990), Haufler et al. (1991) and Taylor et al.
(1990) had made this new material ready for applications among
many fields. The observation of the superconductivity by Hebard
et al. (1991) at Tc = 19 K of potassium-doped
C60 crystal further accelerated the research field.
Within a few years, macroscale amount of metal containing fullerene
[Chai et al. (1991), Shinohara et al. (1992) and
Kikuchi et al. (1993)], higher fullerenes [Kikuchi et
al. (1992) and Achiba & Wakabayashi (1993)] and bucky
tube in Fig. 1.1(c) [Iijima (1991) and Ebbesen & Ajayan (1992)]
were available.

A common technique of the macroscopic
generation of fullerene is the arc-discharge method which is simply
an arc-discharge of graphite electrodes under certain pressure
of helium buffer gas condition proposed by Haufler et al.
(1991). The amount of extracted fullerene compared to the collected
soot can yield up to about 15% under the optimum condition such
as Maruyama et al. (1994b). Usually the generated fullerene
consists of 80% of C60, 15% of C70 and a
small amount of higher fullerene like C76, C78,
C82, C84, .., quite interesting magic numbers.
It is also surprising that once vaporized carbon atoms automatically
form such highly symmetric structures like C60 in the
clustering process. Besides these theoretical interests, it is
required to clarify the generation mechanism in order to find
a more efficient generation method of the higher fullerene or
the metal doped fullerene.
Since the macroscale generation technique
was found accidentally, the formation mechanism of fullerene is
not clear. Several models have been proposed based on experimental
insights. Haufler et al. (1991) described that a growth
of a hexagonal network by successive additions of dimers and trimers
eventually left pentagons as the defect. They claimed that the
pentagons was essential to give the curvature and to decrease
the number of dangling bonds. On the other hand, two neighboring
pentagons should result in too much strain to the network system,
so the Isolated Pentagon Rule (IPR) was assumed. It is interesting
to learn that the smallest fullerene satisfying IPR is C60
and next to the smallest is C70. Quite different precursors
of fullerene structure were proposed, such as a piece of graphitic
sheet [Robertson et al. (1992)] or once grown bucky tube
[Dravid et al. (1993)]. On the other hand, Heath (1992)
proposed a model of clustering sequence from linear chain up to
C10, rings in C10-C20 range,
fullerene at C30. He explained that successive C2
additions followed until the satisfaction of IPR. Wakabayashi
& Achiba (1992) proposed a special model that fullerene was
made of stacking of carbon rings. They could explain magic numbers
of higher fullerenes [Kikuchi et al. (1992) and Achiba
& Wakabayashi (1993)] and isomers of C76, C86
and C84 [Wakabayashi et al. (1993)]. Recently,
the drift tube ion chromatography experiments of laser vaporized
carbon clusters showed the existence of poly-cyclic rings and
the possibility of the annealing of such structure to fullerene
[Helden et al. (1993), Hunter et al. (1993), Clemmer
et al. (1994) and Hunter et al. (1994)].
Although each model can explain some
experimental results, it is difficult to decide which model is
more plausible. We have applied the molecular dynamics method
to simulate the clustering process of carbon atoms in order to
speculate the formation process of fullerene from a different
point of view. Our previous result [Maruyama & Yamaguchi (1995b)]
showed the possibility of simulating the fullerene formation process
from the randomly distributed carbon atoms. However, since the
temperature control was rather arbitrary, the comparison with
the physical phenomena was not easy and no perfect fullerene was
obtained. In this study, we have performed the similar simulations
with more sophisticated temperature control and considered the
importance of cooling rate and annealing.
The classical molecular dynamics
method was employed for the simulation. There were two major difficulties
to overcome in this simulation. One was the assumption of the
many-body potential function for carbon atoms. Since the whole
process involved the chemical reaction and the bonding of carbon
atoms could be varied from sp, sp2 and sp3,
we could not expect a reliable classical formulation of many-body
potential. We employed the potential function developed by Brenner
(1990) and ignored the sophisticated conjugate term, because the
tough and qualitative function was more plausible than the quantitative
but delicate potential function.
Another difficulty was the intrinsic
long time scale of the physical process. The laser-vaporization
supersonic nozzle experiments suggested that the time scale of
the formation of fullerene was about 100 microseconds [Maruyama
et al. (1990)]. Probably, the time scale was longer for the arc-discharge
method. Then, the direct molecular dynamics simulation of full
time scale is far from possible even for the fastest supercomputers.
Our choice was the compression of the time scale, simply by assuming
the high number density of initial carbon atoms. This assumption
forced us the extremely painful modifications of the boundary
conditions and very careful appreciation of the simulated results.
We assumed that the process could be divided into 3 major procedures:
1) Collisions of atoms or clusters with each other which would
result in the growth of cluster or sometimes fragmentation; 2)
Cooling of clusters by the continuous collisions with buffer gas
molecules and radiation; 3) Annealing of each cluster in between
collisions. The high density meant that the rate of procedure
1) was much increased. Then, we assumed the rate of procedure
2) should be increased accordingly. Because the long time scale
of the real system should imply the relatively equilibrium distribution
of kinetic energy to translational, rotational and vibrational
energy, we used the temperature control method which included
the strong tendency toward the equilibrium. Finally, the effect
of procedure 3) was very much underestimated. We tried to estimate
the effect of the annealing in the separate simulation.
2.1 Many-Body Potential Function
We have employed the potential function proposed by Brenner (1990), originally used for his simulation of CH4 CVD (chemical vapor deposition) of the diamond film. This potential was based on the Tersoff's (1986) bond-order expression and was modified to describe the variety of small hydrocarbons, graphite, and diamond lattice. The total energy of the system Eb was described as the sum of the bonding energy of each bond between atom i and j.

where VR(r) and VA(r) were repulsive and attractive force terms, respectively. Morse-type exponential functions with a cut-off function f(r) were used for these functions.



The state of the bonding was expressed through the term B* as the function of angle between bond i-j and each neighboring bond i-k.


where

Since small carbon clusters were not taken into account for Brenner's simulation, the clustering process from small clusters to poly-cyclic bond-network structures could not be simulated [Maruyama & Yamaguchi (1995a)]. In order to compensate it, we have ignored the conjugate-compensation term F which was included in the Brenner's original expression. Constants were as follows.
De = 6.325 eV S = 1.29 = 1.5 Å-1 Re = 1.315 Å
R1 =1.7 Å R2 = 2.0 Å.
= 0.80469 a0 = 0.011304 c0 = 19 d0 = 2.5
Simple Verlet's
method was adopted to integrate the classical equation of motion
with its time step of 0.5 fs.
2.2 Temperature Control Technique
A cluster was defined as a group of carbon atoms inter-connected one another with C-C bonds. Here, the C-C bond was defined between 2 carbon atoms whose distance was smaller than the cut-off distance R2. The kinetic energy of a cluster Cn with n carbon atoms was divided into translational KT, rotational KR and vibrational energy KV. Each energy was expressed as



where m was the mass of the
carbon atom, 












where n
was the number of freedom of each motion of a cluster, and kB
was Boltzmann constant.
As described previously, we tried
to enforce the equilibrium of translational, rotational and vibrational
temperature of the system in order to incorporate the compression
of the time scale by the increase of the number density of carbon
atoms. In this calculation, each temperature of the system was
independently controlled every 0.1 fs through simple velocity
scaling so that the difference between present temperature of
the system and control temperature was reduced to 60 %.
In order to determine the characteristics
of the potential function when applied to fullerene, high temperature
stability of the structure of Buckminsterfullerene C60
[Fig. 1.1 (a)] was examined. Sixty carbon atoms were located at
the equilibrium position of the truncated icosahedron C60
as the initial condition. Then, the vibrational motion was simulated
at various control temperature Tc for 1 ns.
Fig. 3.1 summarizes the observed structure after 1ns at each temperature. For clarity, bond-network structures were expressed as two-dimensional maps for the hollow caged structures in Fig. 3.1 (a-d). When the temperature was below 2500 K, no change of network structure was observed within 1 ns [Fig. 3.1 (a)]. Shaded pentagons should help to check the characteristics of truncated icosahidran, twelve isolated pentagons and 20 hexagons in accordance to IPR. Slight deformation of the network structure was noticeable for Tc = 2600 K in Fig. 3.1 (b). The isomerization by concerted transformations was observed as discussed later. For 2600 K < Tc < 3000 K, transformations occurred more frequently but principally keeping the 5-6 network structure. At Tc = 3000 K, dangling bonds for atoms marked by empty symbols were seen as Fig. 3.1 (c). With these dangling bonds, much more violent and continuous reactions were observed. At Tc = 3200 K, several dangling bonds were moving around the network [Fig. 3.1 (d)]. When the temperature was 3500 K, the caged structure was partially broken with a large hole [Fig. 3.1 (e)], and then, transformation to a chaotic 3-dimensional cluster was observed in Fig. 3.1 (f) at Tc = 4000 K. At Tc = 5000 K, a part of cluster was dissociated from the main body [Fig. 3.1 (g)], and when the temperature was as high as 6000 K, the main body could not keep the size and divided into several small clusters [Fig. 3.1 (h)].


Fig. 3.2 shows the detailed process of the pentagon-migration transformation observed at 2600 K [Fig. 3.1 (b)]. Until 19.5 ps the network structure kept perfect C60. From 19.5 ps through 20 ps, bonds marked A-B and C-D were broken and a new bond A-C was created, leaving dangling bonds on atom B and D [Fig. 3.2 (b)]. Then, B chose atom D as a new bond partner at t = 20.5 ps [Fig. 3.2 (c)]. During this process, the line connecting 4 atoms marked by empty symbol in Fig. 3.2 were merely twisted without the breakage. The whole process could be regulated as a simple migration of pentagons without the change of the total number of pentagons and hexagons. This process known as Stone-Wales transformation or pyracylene rearrangement has been argued as a possible path of isomerization of carbon clusters [Stone & Wales (1986)].
We have shown the similar results
previously [Maruyama & Yamaguchi (1995b)], where the total
energy of the system was kept constant and calculated only for
100 ps. Previous results showed that no isomerization was observed
up to 3000 K. Since the difference of temperature control should
have minor effect in this calculation, the difference of the observation
time should be the reason of the discrepancy. If we assume the
Arrhenius type of reaction rate for the isomerization process,
this discrepancy can be explained that with longer time we have
more chance to see the isomerization process. Since the activation
energy was about 3 eV by this simulation, the rough extrapolation
of the time scale to seconds (109 ns) will give the
order of 1000 K for the onset of the isomerization which is in
good agreement with experimental knowledge. Furthermore, it is
suggested that the compression of time must be accompanied with
the proper increase of temperature in order to reproduce the reasonable
reaction.
The most interesting point about
the formation mechanism of fullerene is how such a hollow caged
structure could be self-assembled. As seen in the previous section,
fullerene structure is extraordinary stable, even though the 2500
K must be over-estimated, and we supposed the high temperature
stability would be the key of the preference of fullerene compared
to diamond or graphite structure. Although we have already demonstrated
that the clustering process of caged structures and flat structures
by using this potential function [Maruyama & Yamaguchi (1995b)],
it was difficult to evaluate the effect of temperature and density
because of the inequilibrium of temperature caused by the previous
temperature control method. Then, we applied a new temperature
control method and calculated the clustering process under the
artificial equilibrium condition.
As an initial condition, 200 carbon
atoms with random velocity were located at random positions in
a 8 nm cubic box with full periodic boundary conditions. Then,
translational, rotational and vibrational temperature of the system
was independently controlled toward a constant temperature Tc
by the technique in the previous section. Several snapshots of
the simulation for Tc = 3000 K are shown in Fig. 4.1.
At 20 ps, most clusters were smaller than C3, while
several chain clusters as large as C10 were observed.
At about t = 60 ps, some of clusters grew to ring or chain structure
with about 12 atoms, and the largest cluster C25 formed
a poly-cyclic network structure. A chaotic 3-dimensional structures
C37, C45, C49 and flat structure
C62 appeared at t = 120 ps, and finally at t = 300
ps, a large tube-like cluster as large as C160 with
hollow caged structure mainly consisted of pentagons and hexagons
was obtained. Through the process, the equilibrium of the temperature
was realized except for the initial 50 ps when the released potential
energy was extremely large compared to the cluster size.


Fig. 4.2 shows the representative
clusters observed for various temperature conditions after 300
ps calculation. For Tc = 1000 K, the cluster had almost
complete 2-dimensional structure though some large rings remained
[Fig. 4.2 (a)]. For Tc = 2000 K as shown in Fig. 4.2
(b), small poly-cyclic rings had coalesced to a graphitic network
structure consisted of 2 flat components and an irregular part
at the middle. When the control temperature was 2600 K, the cluster
constructed a imperfect cage structure with a large hole [Fig.
4.2 (c)]. And for Tc = 3000 K, a hollow caged structure
without large holes was observed [Fig. 4.2 (d)]. For higher temperature
as Tc = 3500 K [Fig. 4.2 (e)], the clusters could not
keep any regular structure and transformed to chaotic 3-dimensional
structure including several carbon atoms with four bonds. For
Tc = 6000 K, the clusters could not keep large size
due to the dissociation process and formed simpler structures
[Fig. 4.2 (f)]. Under very high temperature condition as Tc
= 8000 K [Fig. 4.2 (g)], the clusters could not grew large and
formed some small simple structures such as chains or single rings
up to about C20.
4.2 Precursors in the Reaction
Process
The clustering process was examined
in detail with the special attention to the structure and vibrational
temperature of precursors. The clustering process yielding to
a caged structure for Tc = 3000 K are shown in Fig.
4.3 (a). Precursor clusters smaller than C20 had simple
structure basically constructed of chains and a few rings. The
coalescence of these small pieces resulted in the random chaotic
3-dimensional structure around C40. Then, a rather
complex transformation to a spherical structure of around C50
followed [marked (2) in Fig. 4.3 (a)]. At the same time, another
large cluster C64 had the flat graphitic structure.
The collision of these two clusters [mark (3) in Fig. 4.3 (a)]
and annealing [mark (4) in Fig. 4.3 (a)] resulted in the caged
structure of C160.
The bottom diagram shows the vibrational
temperature Tv and the bond-switching rate Rs
for the marked clusters. The parenthesized mark on top and bottom
panel corresponds in time. Here, the bond switching rate Rs
was defined as the number of bond creation and breakage in a cluster
in 1 ps divided by the number of atoms of the cluster. The small
clusters with larger vibrational energy frequently switched the
bond-network structure, and grew to larger cluster before the
stabilization of the structure [mark (1) in Fig. 4.3 (a)]. The
process marked as (2) in Fig. 4.3 (a) shows the rapid annealing
and decrease of the bond-switching rate toward C50.
The bond-switching rate is rather small after the middle of the
process marked (2) compared to the smaller clusters, implying
that the gradual annealing of semi-caged cluster.

On the other hand, the clustering
process yielding to a graphitic structure was very simple as shown
in Fig. 4.3 (b). The precursors always formed flat structure and
extended the size in 2-dimensional direction at Tc
= 1000 K. During the whole process, the clusters had low vibrational
temperature and changed the network structure only in the case
of the collision and only around the contact point in order to
organize the flat structure.





4.3 Transition of Cluster Size
Distribution
Although the selectivity of the caged
structure in the certain temperature range is explained in the
previous section, it is still unknown why C60 is so
preferable than other sized fullerene. About 80 % of generated
fullerene are C60 for typical arc-discharge method.
On the other hand, the percentage of C60 in fullerene
generated in laser-vaporization cluster source is usually less
than 0.1 %. The key mechanism of C60 selection must
explain this difference. We considered that the annealing of clusters
should give a hint to this question. The simulation shown in Fig.
4.3 (a) cannot be regarded as successful, because the final collisions
of C50 size range clusters have made too large cluster,
C160.
In order to estimate the time scale
for annealing, we have considered the collision rate and collisional
cross section of each cluster. The transition of cluster size
distribution for Tc = 3000 K is shown in Fig. 4.4.
Here, the time traces of typical large clusters are described.
Long vertical lines apparent in Fig. 4.4 mean the collision free
period. These long lines appeared when the cluster size grew to
about C40 - C60 especially with 3-dimensional
structure. This is related to the decrease of the collisional
cross-section by the transformation to the compact 3-dimensional
structure. As a consequence these 3-demensional clusters can get
enough time for annealing toward the perfect fullerene structure
which would be stable enough to reject further reactions even
with later collisions.


Considering the time scale compression
in this simulation, the time scale of the annealing process appeared
in Fig. 4.4 can correspond to tens of micro-seconds in practice.
In order to evaluate the effect of annealing process, we have
picked up an imperfect C60 caged cluster obtained in
our previous similar simulation [Maruyama & Yamaguchi (1994a)],
and simulated the annealing process without collision. The temperature
was controlled at Tc = 3000 K for first 8 ns to promote
rapid annealing, then set at Tc = 2500 K for about
50 ns afterwards. The transition of bond-network structure is
summarized in Fig. 5.1. Here, shaded and empty faces are pentagons,
and hexagons, respectively, heptagonal faces are marked as 7,
empty symbols are atoms with dangling bonds. The initial cluster
included 4 dangling bonds and 8 heptagons [Fig. 5.1 (a)]. The
number of these defects gradually decreased until 15 ns [Fig.
5.1 (c)], where all dangling bonds were terminated and no heptagons
remained. A clear correspondence can be seen in the potential
energy per atom in bottom picture. Then, the bond-network could
gradually changed through Stone-Wales transformation without the
inclusion of heptagons or dangling bonds. However, there were
rather violent reconstruction of whole network structures at around
18 ns [Fig. 5.1 (c)] and 34 ns. There were only successive Stone-Wales
transformations after about 37 ns [Fig. 5.1 (e)] toward the final
perfect Buckminsterfullerene C60 in Fig. 5.1 (f). We
believe that the violent reconstruction is due to a little too
high temperature of the simulation and is not necessary for the
real physical annealing process.
A molecular dynamics simulation of
the clustering process starting from randomly distributed carbon
atoms yielded to the imperfect fullerenes under certain high temperature
control. On the other hand, flat graphitic sheets were generated
under low temperature condition. The caged structure was shaped
through the annealing of chaotic 3-dimensional precursors around
C50 with small surface area, in contrast with the consecutive
growth mechanism of graphitic sheet. The 3-dimensional clusters
around C50 could form a more sophisticated structure
through their long lifetime due to the small collisional cross
section. Although the simplified potential and the time-scale
compression technique made it difficult to compare the simulation
with experimental results, we could demonstrate the formation
of fullerene completely through the self-assembly of carbon atoms.
Furthermore, the possible annealing process to the perfect Buckminsterfullerene
C60 was demonstrated. These results would indicate
the possible path of clustering to fullerene.
B*: Normalized bond order function
D: Potential well depth
Eb: Bonding energy
f: Cut-off function
K: Kinetic energy
kB: Boltzmann constant
m: Mass of carbon
n: Number of molecules of a cluster
Rs: Bond switching rate
R: Lattice constant
rij: Distance between atom i and atom j
r: Position vector
S: Potential parameter
T: Temperature
Tc: Control temperature
t: Time
V: Potential function
v:
Velocity vector
Greek Symbols
: Potential parameter
: Potential parameter
: Number of freedom of cluster motion
: Angle
Subscripts
A: Attractive
e: Equilibrium
V: Vibrational
R: Rotational or repulsive
T: Translational
We thank Professor Emeritus Susumu
Kotake at the University of Tokyo for his kind discussions during
this study. This work was supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Science and Culture,
Japan.
Achiba, Y. & Wakabayashi, T., 1993, "Stability, Structures and Hypothetical Growth Mechanism of Carbon 5/6 Network," Z. Phys. D, 26, 69-73.
Brenner, D. W., 1990, "Empirical Potential for Hydrocarbons for Use in Simulating the Chemical Vapor Deposition of Diamond Films," Phys. Rev. B, 42-15, 9458-9471.
Chai, Y. et al., 1991, "Fullerenes with Metals Inside," J. Phys. Chem., 95, 7564-7568.
Clemmer, D. E. et al., 1994, "Gas-Phase Self-Assembly of Endohedral Metallofullerenes," Nature, 367, 718-720.
Dravid, V. P. et al., 1993, "Buckytubes and Derivatives: Their Growth and Implications for Buckyball Formation," Science, 259, 1601-1604.
Ebbesen, T. W. & Ajayan, P. M., 1992, "Large-Scale Synthesis of Carbon Nanotubes," Nature, 358, 220-222.
Haufler, R. E. et al., 1991, "Carbon Arc Generation of C60," Mat. Res. Soc. Symp. Proc., 206, 627-638.
Heath, J. R., 1992, "Synthesis of C60 from Small Carbon Clusters, A Model Based on Experiment and Theory," Fullerenes, Ed. G. S. Hammand & V. J. Kuck, American Chemical Society (Washington, D. C.), 1-23.
Hebard, A. F. et al., 1991, "Superconductivity at 18K in Potassium-Doped C60," Nature, 350, 600-601.
Iijima, S., 1991, "Helical Microtubules of Graphitic Carbon," Nature, 354, 56-58.
Helden, G. et al., 1993, "Experimental Evidence for the Formation of Fullerenes by Collisional Heating of Carbon Rings in the Gas Phase," Nature, 363, 60-63.
Hunter, J. et al., 1993, "Annealing C60+: Synthesis of Fullerenes and Large Carbon Rings," Science, 260, 784-786.
Hunter, J. M. et al., 1994, "Annealing Carbon Cluster Ions: A Mechanism for Fullerene Synthesis," J. Phys. Chem., 98-7, 1810-1818.
Kikuchi, K. et al., 1992 "Isolation and Identification of Fullerene Family: C76, C78, C82, C84, C90 and C96," Chem. Phys. Lett., 188-3,4, 177-180.
Kikuchi, K. et al., 1993 "Isolation and Characterization of the Metallofullerene LaC82," Chem. Phys. Lett., 216-1,2, 23-26.
Krtschmer, W. et al, 1990, "Solid C60: a new form of carbon," Nature, 347, 354-358.
Kroto, H. W. et al., 1985, "C60: Buckminsterfullerene," Nature, 318-6042, 162-163.
Maruyama, S. et al., 1990, "Direct Injection Supersonic Cluster Beam Source for FT-ICR Studies of Clusters," Rev. Sci. Instrum., 61-12, 3686-3693.
Maruyama, S. et al., 1991, "Thermionic Emission from Giant Fullerenes," Z. Phys. D, 19, 409-412.
Maruyama, S. et al., 1994a, "A Molecular Dynamics Simulation for the Formation Mechanism of Fullerene," Proc. 31st National Heat Transfer Conf., 940-942.
Maruyama, S. et al., 1994b, "The Effect of Helium Convection on the Generation Process of Fullerene," Proc. 31st National Heat Transfer Conf., 943-945.
Maruyama, S. & Yamaguchi, Y., 1995a, "A Molecular Dynamics Simulation for the Formation Process of Fullerene (Part 2)," Proc. 32nd National Heat Transfer Conf., 839-840.
Maruyama, S. & Yamaguchi, Y., 1995b, "A Molecular Dynamics Simulation for the Formation Mechanism of Fullerene," Therm. Sci. & Engng., 3-3,105-109.
O'Brien S. C. et al., 1988, "Photophysics of Buckminsterfullerene and Other Carbon Cluster Ions," J. Chem. Phys., 88-1, 220-230.
Robertson, D. H. et al., 1992, "On the Way to Fullerenes: Molecular Dynamics Study of the Curling and Closure of Graphitic Ribbons," J. Phys. Chem., 96, 6133-6135.
Rohlfing, E. A. et al., 1984, "Production and Characterization of Supersonic Carbon Cluster Beams," J. Chem. Phys., 81-7, 3322-3330.
Shinohara, H. et al., 1992 "Mass Spectroscopic and ESR Characterization of Soluble Yttrium-Containing Metallofullerenes YC82 and Y2C82," J. Phys. Chem., 96, 3571-3573.
Smalley, R. E., 1993, "From Dopyballs to Nanowires," Materials Science and Engineering, B19, 1-7.
Stone, A. J. & Wales, D. J., 1986, "Theoretical Studies of Icosahedron C60 And Some Related Species" Chem. Phys. Lett., 128, 501-503.
Taylor, R. et al., 1990, "Isolation, Separation, and Characterization of the Fullerenes C60 and C70: The Third Form of Carbon," J. Chem. Soc. Chem. Communications, 1423, 1423-1425.
Tersoff, J., 1986, "New Empirical Model for the Structural Properties of Silicon," Phys. Rev. Lett., 56-6, 632-635.
Wakabayashi, T. & Achiba, Y., 1992, "A Model for the C60 and C70 Growth Mechanism," Chem. Phys. Lett., 190-5, 465-468.
Wakabayashi, T. et al., 1993, "A Selective Isomer Growth of Fullerenes," Chem. Phys. Lett., 201-5,6, 470-474.